Introduction
Eptacog beta [Sevenfact®, coagulation factor VIIa (recombinant)-jncw] (HEMA Biologics and LFB) is a human rFVIIa variant indicated for the treatment and control of bleeding events (BEs) in adults and adolescents with hemophilia A or B and inhibitors. In a phase 3 clinical trial (PERSEPT 1, NCT02020369) two initial dose regimens of eptacog beta (75 µg/kg q3h; and 225 µg/kg followed by 75 µg/kg q3h after 9 hours if necessary) demonstrated hemostatic efficacy at 12 hours (82% and 91% respectively) in inhibitor-related mild or moderate bleeding with a very low incidence of rebleeding. Two additional phase 3 trials have been completed and a phase 4 trial is planned.
Emicizumab (Hemlibra®, Genentech) is a bispecific antibody indicated for prophylaxis in persons with hemophilia A (with or without inhibitors) to reduce the frequency of bleeding. Emicizumab cannot treat a BE; instead, persons with hemophilia A or B with inhibitors require the use of a bypassing agent (BPA). In clinical trials and post-marketing surveys, thromboembolic events (TEs), including thrombotic microangiopathy have been observed with the concomitant use of aPCC (Feiba®, Shire) (>100 U/kg/day for >1 day) and emicizumab. These TEs have not been observed with emicizumab and eptacog alfa (rFVIIa, NovoSeven® RT, Novo Nordisk) alone.
Aims
To determine the in vitro procoagulant activity of eptacog beta alone and in combination with emicizumab utilizing a thrombin generation (TG) assay with hemophilia A (HA) and hemophilia A inhibitor (HAI) plasma. The in vitro TG attributed to the combined products will be used to gauge the potential safety and efficacy of eptacog beta for the treatment of BEs in persons with hemophilia A with inhibitors managed with this type of prophylaxis.
Methods
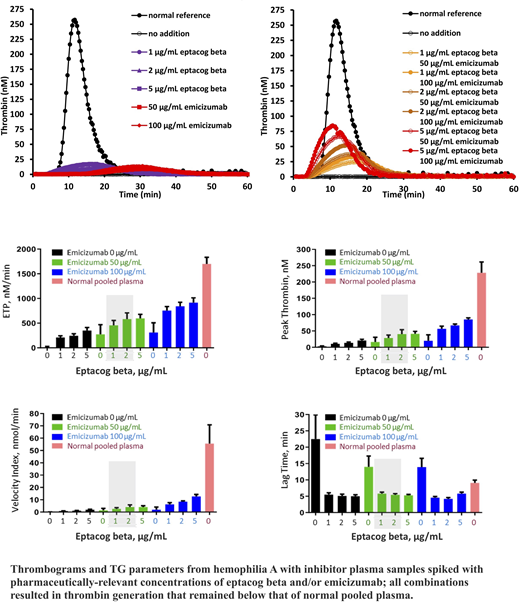
TG assays were performed according to the method developed by Hemker et al using a Fluoroskan Ascent fluorometer (Thermo Labsystems). TG curves, peak thrombin, endogenous thrombin potential (ETP), lag time and velocity index were determined for severe HA plasma (10 samples) and HAI plasma (4 high titer samples) using both platelet-poor plasma (phospholipid and tissue factor initiator) and platelet-rich plasma (tissue factor initiator).
Eptacog beta concentrations (1 µg/mL and 2 µg/mL) were selected to reflect the approximate peak plasma levels observed in the phase 1b trial [NCT01708564; 75 µg/kg dose (0.72 µg/mL) and 225 µg/kg dose (1.9 µg/mL)]; eptacog beta (5 µg/mL) was also examined. Emicizumab (50 µg/mL and 100 µg/mL) concentrations reflect the approximate maximum steady state levels observed when dosed at 1.5 mg/kg (55 µg/mL) and 6 mg/kg (67 µg/mL).
Results
When no procoagulants were added to HA and HAI plasma, peak thrombin, ETP and velocity index were reduced, and lag time was increased compared to normal pooled plasma (NPP). Addition of emicizumab (50 or 100 µg/mL) and/or eptacog beta (1, 2, or 5 µg/mL) induced a concentration-dependent increase in peak thrombin, ETP, and velocity index. Notably, the effect was lower than that seen with normal plasma: peak thrombin, ETP and velocity index for all combinations and single agents in HAI plasma were significantly lower than those observed in NPP (P<0.001, P-values from t-tests comparing NPP mean TG parameters with the corresponding parameters from HAI plasma). A slight shortening of the lag time below that observed in NPP was observed when both eptacog beta and emicizumab were jointly present in the assay.
Conclusions
These in vitro data show that clinically relevant combinations of eptacog beta and emicizumab result in a concentration-dependent increase in thrombin generation that remains below that observed in normal pooled plasma. This observation is comparable to that reported for eptacog alfa/sequence identical analog emicizumab (SIA-emicizumab), which was used to explain the clinical safety of the combination and rationalize the lack of TEs. The similarity of these results further suggests that eptacog beta could be used as an alternate BPA for the treatment of a breakthrough BE in inhibitor patients utilizing emicizumab prophylaxis. A clinical trial to investigate this observation is planned.
Pipe:Siemens: Research Funding; Medical and Scientific Advisory Council to the National Hemophilia Foundation; Medical Advisory Board to World Federation of Hemophilia: Membership on an entity's Board of Directors or advisory committees; Apcintex, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, F. Hoffmann-La Roche Ltd/Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, uniQure: Consultancy. Recht:Spark: Research Funding; BioMarin: Research Funding; Genentech: Consultancy, Other: personal fees, Research Funding; Pfizer: Consultancy, Other: personal fees; uniQure: Consultancy, Other: personal fees, Research Funding; Takeda: Consultancy, Other: personal fees, Research Funding; CSL Behring: Consultancy, Other: personal fees; Novo Nordisk: Consultancy, Other: personal fees, Research Funding. Callaghan:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biomarin: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Site Investigator/sub-I Clinical Trial, Speakers Bureau; Alnylum: Current equity holder in publicly-traded company; Hema Biologics: Honoraria, Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Other, Speakers Bureau; Roche/Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Site Investigator/sub-I Clinical Trial, Speakers Bureau; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Spark: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Grifols: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sancillio: Other; Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Site Investigator/sub-I Clinical Trial, Research Funding; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau. Sidonio:Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Biomarin: Consultancy, Honoraria, Speakers Bureau; Uniqure: Consultancy, Honoraria; Spark: Consultancy, Honoraria; Grifols: Research Funding. Grandoni:LFB: Ended employment in the past 24 months. Duretz:LFB: Current Employment. Bonzo:International Association for Statistical Computing: Other; International Statistics Institute: Other; American Statistical Association: Other; LFB USA, Inc.: Current Employment. Plantier:LFB: Current Employment. Evans:LFB: Current Employment. Mitchell:HEMA Biologics: Consultancy. Hermans:Bayer: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Shire, a Takeda company: Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Research Funding, Speakers Bureau; Biogen: Consultancy, Speakers Bureau; CAF-DCF: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; LFB: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Octapharma: Consultancy, Speakers Bureau; Kedrion: Speakers Bureau; EAHAD: Other; WFH: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal